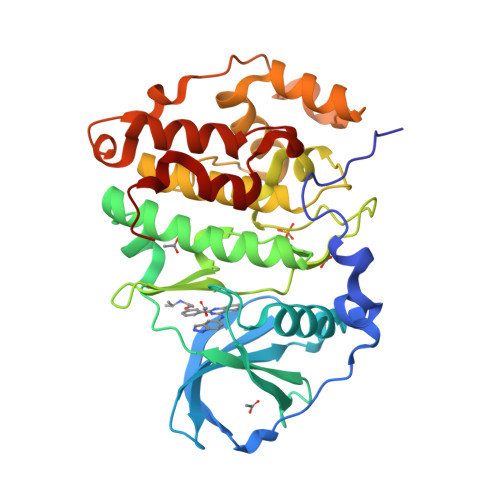
Crystal structure of the Rho-associated coiled-coil kinase 2 inhibitor belumosudil bound to CK2 alpha.
Brear, P., Hyvonen, M.(2022) Acta Crystallogr F Struct Biol Commun 78: 348-353
- PubMed: 36189718
- DOI: https://doi.org/10.1107/S2053230X22008767
- Primary Citation of Related Structures:
7Z39 - PubMed Abstract:
The small molecule belumosudil was initially identified as a selective inhibitor of Rho-associated coiled-coil kinase 2 (ROCK2) and has recently been approved for the treatment of graft-versus-host disease. However, recent studies have shown that many of the phenotypes displayed upon treatment with belumosudil were due to CK2α inhibition. CK2α is in itself a very promising therapeutic target for a range of conditions and has recently been put forward as a potential treatment for COVID-19. Belumosudil presents a promising starting point for the development of future CK2α inhibitors as it provides a safe, potent and orally bioavailable scaffold. Therefore, several of the major hurdles in drug development have already been overcome. Here, the crystal structure of belumosudil bound to the ATP site of CK2α is presented. This crystal structure combined with modelling studies further elucidates how belumosudil could be developed into a selective and potent CK2α or ROCK2 inhibitor.
Organizational Affiliation:
Department of Biochemistry, University of Cambridge, 80 Tennis Court Road, Cambridge, United Kingdom.